Abstract
Introduction: Acute Myeloid Leukemia (AML) is a malignant disease of the bone marrow that can arise from a premalignant condition called clonal hematopoiesis of indeterminate potential (CHIP). Mutations in Serine and Arginine-rich Splicing Factor 2 (SRSF2) are detected in CHIP and mediate a high risk for AML development. Here we used CRISPR/Cas9-mediated genome engineering to introduce a heterozygous SRSF2P95H mutation into primary human hematopoietic stem and progenitor cells (HSPCs) and investigated its functional consequences using both in vitro and in vivo assays.
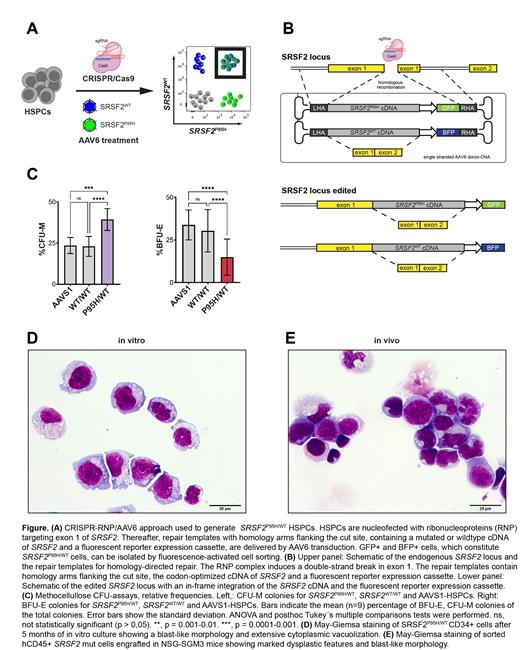
Methods: We used CRISPR/Cas9 technology to introduce a heterozygous mutant (mut) SRSF2P95H into the endogenous SRSF2 gene locus of healthy cord blood HSPCs. Our approach is based on homologous recombination using DNA repair templates delivered by adeno-associated virus serotype 6 (AAV6) (Figure A). This allows for targeted in-frame integration of mut and/or wildtype (WT) SRSF2 cDNA under the control of the endogenous SRSF2 promoter. Notably, an integrated fluorescent reporter enables the isolation and tracking of heterozygously mutated HSPCs (Figure B). Methylcellulose colony and long-term competition assays of SRSF2 mut and WT HSPCs were performed in vitro. Cells were analyzed by flow cytometry and characterized cytomorphologically. In addition, bulk RNA-seq analyses were performed to characterize differential gene expression and abnormal splicing events. Xenotransplantation into NSG-SGM3 mice was performed in order to assess stem cell characteristics and the in vivo leukemogenic potential of SRSF2 mut HSPCs. Finally, we investigated the mutation-specific effect of the splicing inhibitor Indisulam to determine if SRSF2 mut cells are particularly vulnerable to splicing inhibition.
Results: Colony assays (n=9) revealed impaired erythroid and increased monocytic differentiation of SRSF2 mut HSPCs. Quantification of colonies showed a lower frequency of erythroid BFU-E in SRSF2 mut compared to SRSF2 WT HSPCs (mean ± SD; 33.3 ± 12.5% vs. 17.4 ± 10.8%, p=0.00002). In contrast, the frequency of myeloid CFU-M colonies was higher in SRSF2 mut HSPCs compared to SRSF2 WT HSPCs (38.3 ± 7.3% vs. 22.6 ± 6.8%, p = 0.0003) (Figure C). Long-term in vitro competition assays revealed an outgrowth of SRSF2 mut over WT cells in 2 out of 7 donors. Strikingly, after three months of in vitro culture, in one donor, the SRSF2 mut cells developed a blast-like morphology with strong CD34 expression (Figure D). To assess stem cell characteristics and the leukemogenic potential in vivo, we transplanted SRSF2 mut HSPCs from 4 different donors into immunodeficient NSG-SGM3 mice (n=11). SRSF2 mut cells showed a myeloid-skewed engraftment. Cytomorphologic analysis of long-term engrafted SRSF2 mut myeloid cells revealed dysplastic changes such as nuclear abnormalities and extensive cytoplasmic vacuolization. In 4 out of 11 xenografts, human engraftment substantially increased over time with a parallel outgrowth of the SRSF2 mut clone and the appearance of blast-like cells resembling transformation into myeloid leukemia (Figure E). Comparative RNA-seq analysis identified 138 differentially spliced genes, with exon skipping being the dominant altered splicing type. Gene ontology (GO) analysis on differentially expressed genes revealed "Acute Myeloid Leukemia" among the most enriched terms (p-val = 8.2E-07, min FDR = 1.486E-04). When testing the SRSF2-mutation specific effect of the splicing inhibitor Indisulam, SRSF2 mut HSPCs show a significantly lower IC-50 than WT cells (977nM vs. 3574 nM). Strikingly, in competition- and CFU-assays, Indisulam preferentially eradicates SRSF2 mut hematopoietic cells, while sparing WT cells.
Conclusion: Using our CRISPR/Cas9 approach, we can successfully introduce heterozygous SRSF2P95H mutants in primary human HSPCs. Mutant SRSF2P95H leads to increased monocytic differentiation, impaired erythroid differentiation, and phenocopy SRSF2P95H driven diseases in patients. Importantly, we show for the first time that the SRSF2 mutation alone is sufficient to induce dysplastic features and even transform healthy human HSPCs into AML-like blasts. Our model allows the identification and therapeutic investigation of specific cellular vulnerabilities caused by SRSF2 mutations and highlights Indisulam as a potential compound to specifically treat individuals carrying a SRSF2 mutation.
Ediriwickrema: Nanosive SAS: Patents & Royalties. Greinix: Novartis: Consultancy; Celgene: Consultancy; Takeda: Consultancy; Sanofi: Consultancy; Therakos: Consultancy. Sill: Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Zebisch: Celgene: Consultancy, Honoraria; AbbVie: Consultancy; Novartis: Consultancy. Majeti: BeyondSpring Inc.: Membership on an entity's Board of Directors or advisory committees; CircBio Inc.: Membership on an entity's Board of Directors or advisory committees; Kodikaz Therapeutic Solutions Inc.: Membership on an entity's Board of Directors or advisory committees; Coherus Biosciences: Membership on an entity's Board of Directors or advisory committees; Acuta Capital Partners: Consultancy; Gilead: Patents & Royalties: inventor on a number of patents related to CD47 cancer immunotherapy licensed to Gilead Sciences, Inc.. Reinisch: Pfizer: Consultancy; Celgene: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal